ACS Energy Lett.:钴-卟啉MOFs光催化乙炔制乙烯研究
使用最先进的热加氢技术从乙炔污染物中净化粗乙烯需要高温、H2气体的外部进料和贵金属催化剂,不仅成本高、能源密集,而且容易过度加氢生成乙烷。本文报道了利用金属有机框架(MOF) Co-PCN-222光催化乙炔半加氢制乙烯的研究。在纯乙炔气氛下,体系的总转化率为1.6 mol乙烯/g Co,并保持1周的催化活性。在乙炔/乙烯混合气氛(工业相关条件)下,该体系在87 h后乙炔转化率接近100%,乙烯对乙烷的选择性>99.9%。与均相催化剂相比,MOF的多相性质具有优势,即易于回收和寿命延长。这些特性在选择性和可持续性方面也比目前的热催化加氢技术有很大的优势。虽然MOFs在学术上已经被用于乙炔和乙烯的物理分离,但这是第一次证明MOFs作为乙炔选择性光催化转化为乙烯的催化剂。
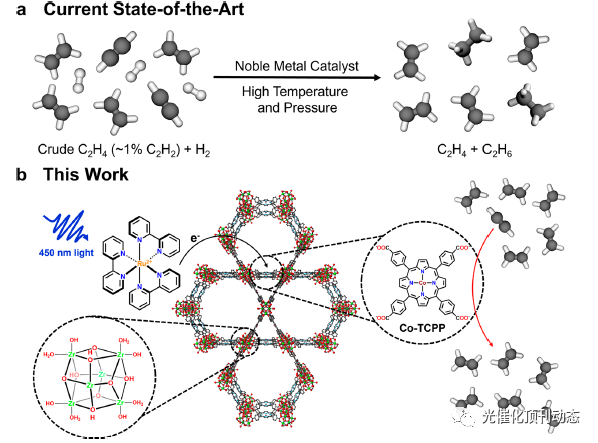
Figure 1. (a) Current state-of-the-art technology for acetylene to ethylene conversion, which involves hydrogenation of acetylene using a noble metal catalyst and H2 at high temperatures. (b) The MOF-based photocatalytic scheme laid out in this work, in which Ru(bpy)3 2+ is used to photosensitize non-noble Co catalyst supported by the Zr-based PCN-222 framework, which reduces acetylene to ethylene photocatalytically at room temperature and with high conversion and selectivity.
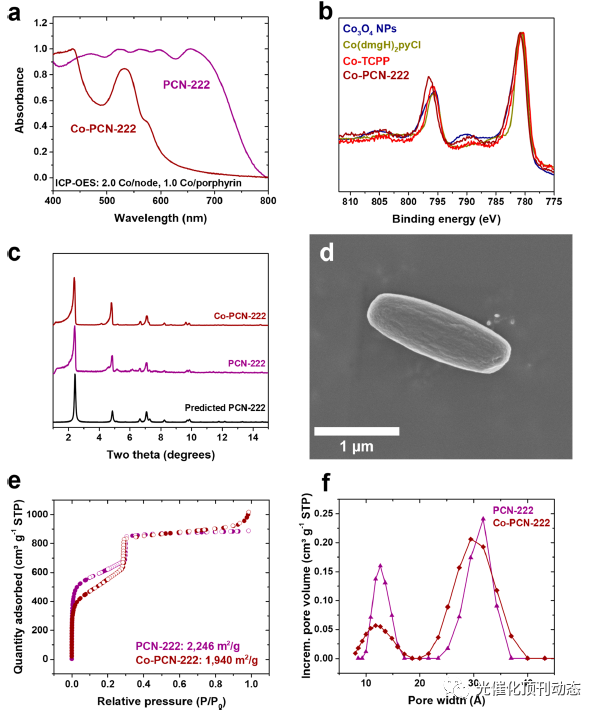
Figure 2. (a) DR−UV−vis of PCN-222 (purple) and Co-PCN-222 (maroon). (b) XPS spectrum of Co-PCN-222 (maroon) and, for comparison, Co3O4 nanoparticles (navy), Co(dmgH)2pyCl (yellow), and Co-TCPP linker (red). (c) Predicted (black) and experimental PXRD patterns of PCN-222 (purple) and Co-PCN-222 (maroon). (d) SEM image of Co-PCN-222. (e) N2-sorption isotherms of PCN-222 (purple) and Co-PCN-222 (maroon). (f) Pore size distributions of PCN-222 (purple) and Co-PCN-222 (maroon).
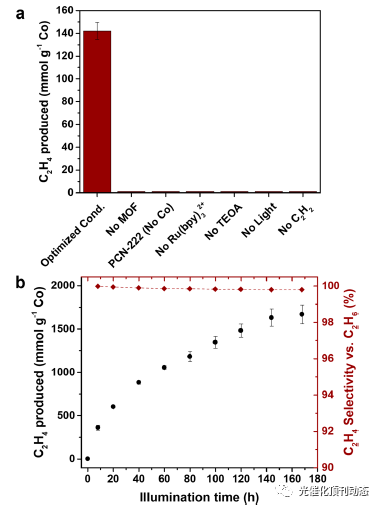
Figure 3. (a) Total ethylene production for the “optimized” reaction mixture containing 2.5 mM Ru(bpy)3 2+, 0.8 mg (0.15 mM) of Co-PCN-222, and 1.25 M TEOA in acetonitrile under C2H2 (≥99.5 vol %) irradiated (450 nm, 140 mW·cm−2) for 4 h, and for reaction mixtures that differ from the optimized conditions as indicated by the axis labels. (b) Total ethylene production and selectivity as a function of irradiation time (450 nm, 140 mW· cm−2) for the Ru(bpy)3 2+/Co-PCN-222 system containing 10 mM Ru(bpy)3 2+, 0.8 mg of Co-PCN-222, and 1.25 M TEOA in acetonitrile under C2H2 (≥99.5 vol %). Error bars represent the standard deviations for at least three separate experiments.
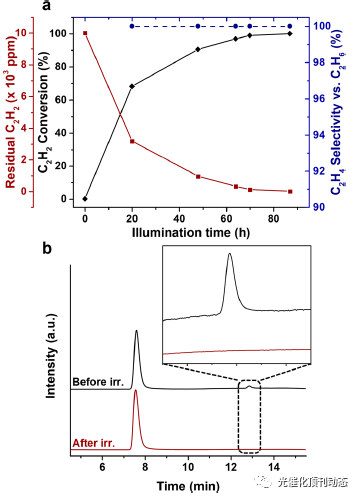
Figure 4. Performance of the Ru(bpy)3 2+/Co-PCN-222 system containing 8 mg (1.5 mM) of Co-PCN-222, 10 mM Ru(bpy)32+, and 1.25 M TEOA under a C2H2/C2H4 mixture (1 vol % C2H2, 30 vol % C2H4, He balance). (a) Plot of acetylene conversion (%), residual acetylene (ppm), and ethylene selectivity vs ethane (%) as a function of irradiation time. (b) Gas chromatograms (the elution order is C2H4, C2H6, C2H2) detected with flame ion detection before and after irradiation (450 nm, 140 mW·cm−2) for 87 h. The inset is a magnified view of the acetylene peak in the chromatogram before and after illumination.
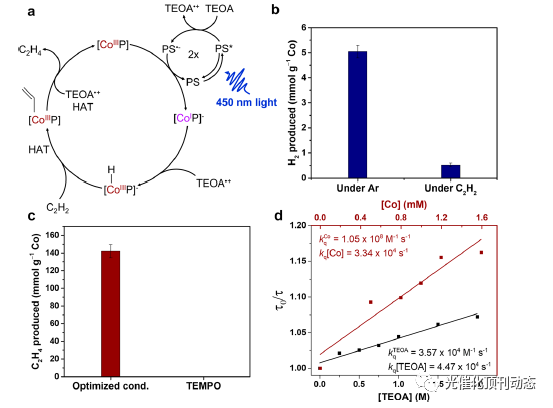
Figure 5. (a) Proposed catalytic mechanism for the photocatalytic reduction of acetylene to ethylene using Co-PCN-222. (b) Comparison of H2 evolution by the Ru(bpy)3 2+/Co-PCN-222 system under acetylene or Ar. (c) Comparison of ethylene production by the /Co-PCN-222 system under optimized conditions or in the presence of 200 molar equiv of TEMPO with respect to Co-PCN-222. (d) Stern−Volmer plot of Ru(bpy)3 2+ luminescence quenching in the presence of Co-PCN-222 (maroon) and TEOA (black).
Selective Photocatalytic Reduction of Acetylene to Ethylene Powered by a Cobalt-Porphyrin Metal−Organic Framework
声明:本文仅用于学术文章转载分享,不做盈利使用,如有侵权,请及时联系小编删除。




