Manufacturing Process Development for Belzutifan
Org. Process Res. Dev. 2022, 26, 525. DOI: 10.1021/acs.oprd.1c00232
◆The oxidation process used in the clinical supply route employed DMSO as the oxidant and solvent
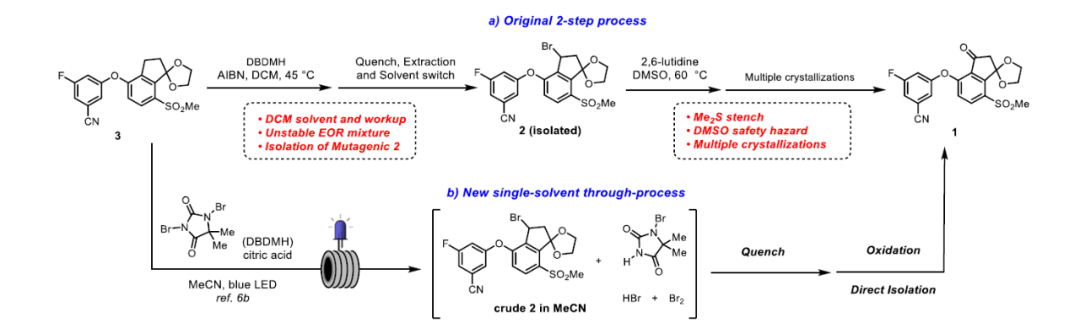
◆An ideal solution would be direct C−H oxidation of ketal 3 to give ketone 1, which would eliminate the mutagenic bromide 2 and one chemical step from the synthesis. However, after evaluating several sets of conditions, we were unable to discover a tractable lead because of competitive oxidation or deprotection of the ketal moiety.
◆An alternative strategy sought to identify a significantly improved reagent system for the oxidation step to address the safety and stench issues and integrate it with the prior bromination step as a single-solvent through-process, thereby obviating the need to isolate bromide 2.
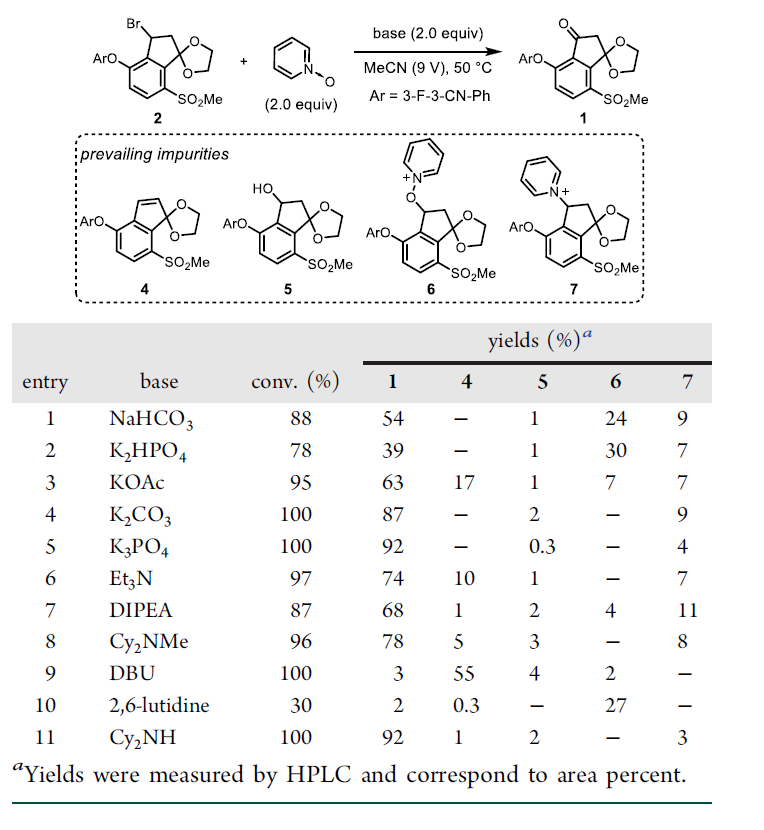
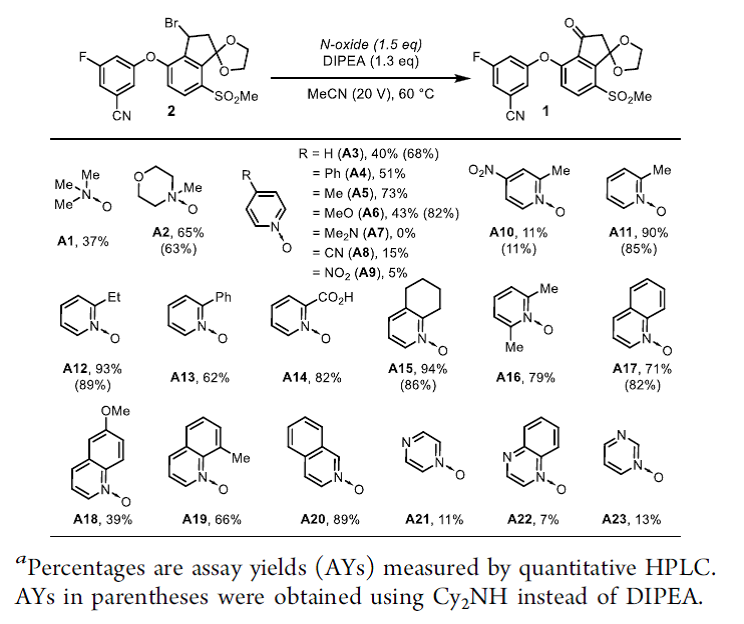
◆DIPEA was chosen for further optimization because it offered minimal amounts of impurities 4 and 5. With DIPEA, we evaluated 23 commercially available N-oxides
◆We reasoned that 2-substituted pyridine N-oxides might be promising reagents since the pyridine byproduct would be more sterically hindered and slower to engage in the undesired substitution with 2.
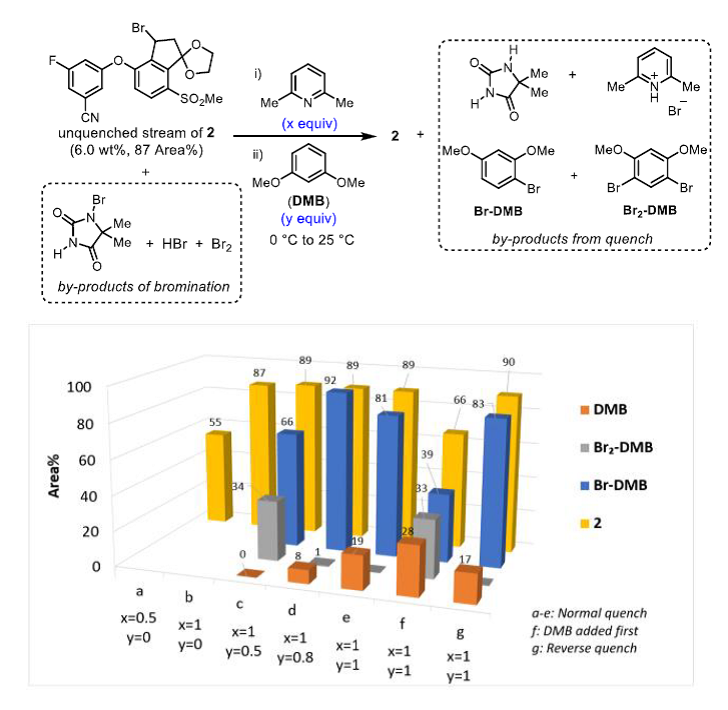
◆The quenching conditions involving the use of neat 2,6-lutidine and 1,3-dimethoxybenzene. The presence of 2,6-lutidine neutralized any trace of HBr present in the reaction stream, and 1,3- dimethoxybenzene reacted with any residual electrophilic bromine (Br+).
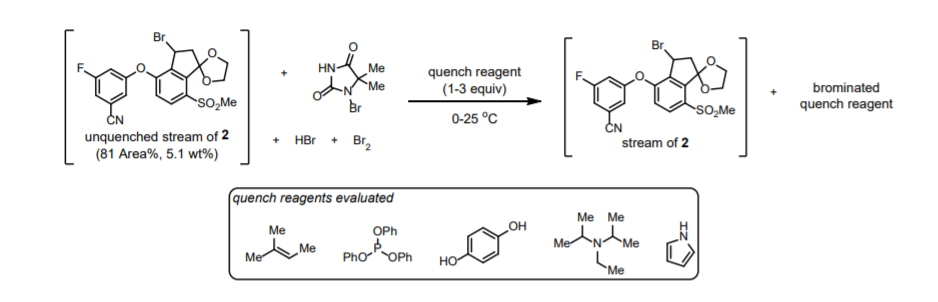
◆The order of addition for the quench was important, since charging DMB before 2,6-lutidine afforded a mixture of DMB, Br-DMB, and Br2-DMB and decomposition of 2.
◆Reverse addition of the crude bromination end-of-reaction (EOR) stream into a preformed solution of DMB and lutidine in MeCN further controlled the quench speciation and stability of 2 robustly on scale