吴明铂/吴文婷ACS Catal:在Au-Pd /ZnO光催化选择性氧化甲烷制甲醇

第一作者中国石油大学QiangZhou,通讯作者中国石油大学吴明铂、吴文婷在《ACS Catalysis》发表题为《Selective Photocatalytic Oxidation of Methane to Methanol by Constructing a Rapid O2 Conversion Pathway over Au−Pd/ZnO》重要成果!
在温和条件下,甲烷光催化氧化制甲醇是一个有吸引力的过程,然而在同时实现高转化率和高选择性方面面临着重大挑战。为此,作者提出了一种通过合理设计Au-Pd /ZnO光催化剂,跳过水溶性H2O2,避免H2O2扩散,从O2 (O2→*OOH→•OH)直接快速生成羟基自由基(•OH)的策略。对于室温下光催化甲烷氧化,优化后的1.0% AuPd0.5/ZnO催化剂上CH3OH的产率和选择性分别高达81.0 μmol·h-1和88.2%,超过了大多数已报道的体系。Au-Pd合金的形成改善了*OOH中O2的吸附和O-O键的断裂,促进了•OH的高效直接生成,提高了CH3OH的收率和选择性。本文的研究成果为设计高效选择性光催化甲烷氧化和O2利用的复合光催化剂提供了指导。
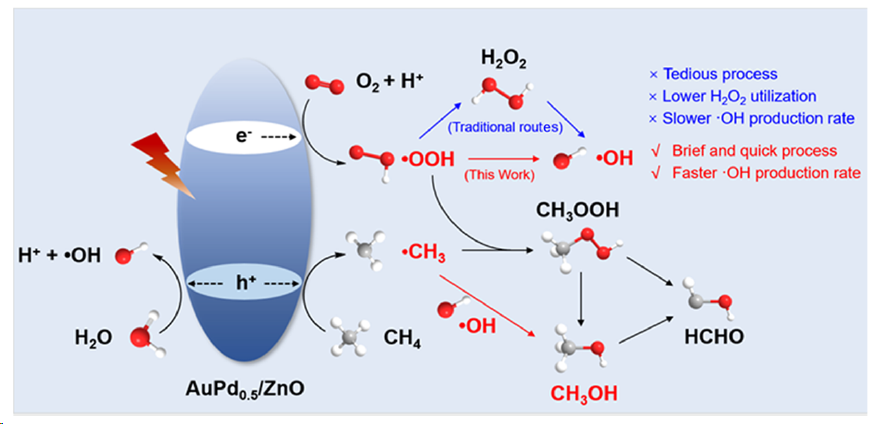
Scheme 1. Mechanism of Photocatalytic CH4 Oxidation on AuPd0.5/ZnO.
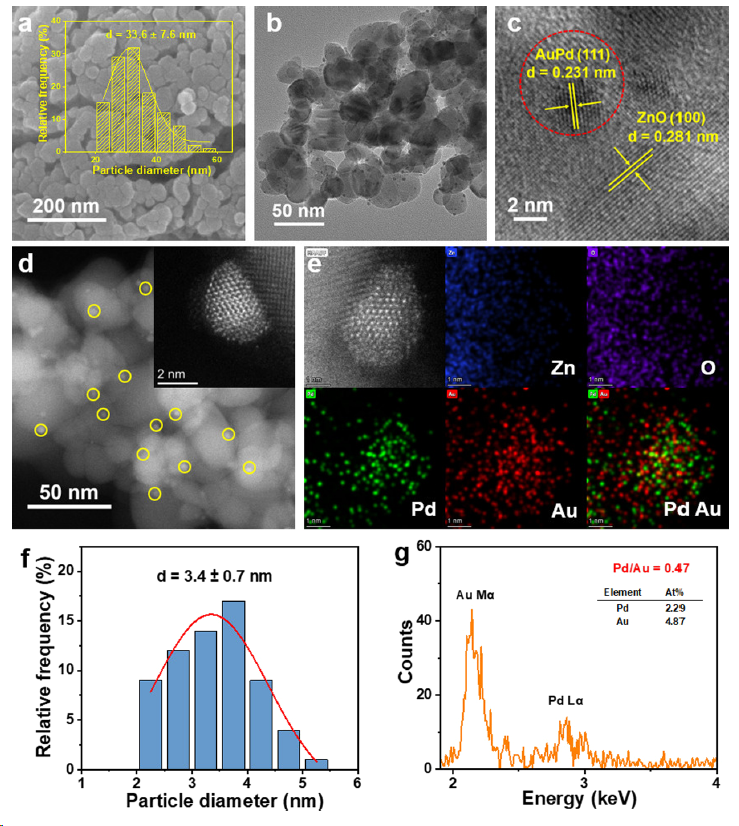
Figure 1. Morphology characterizations of AuPd0.5/ZnO. (a) SEM image, (b) TEM image, (c) HRTEM image, (d) HAADF-STEM and (e) EDSmapping images of AuPd0.5/ZnO photocatalyst. (f) The particle size distribution and (g) EDX spectra of Au−Pd nanoparticles. The insets of parts a and (d) show the particle size distribution of ZnO substrate and HAADF-STEM image of Au−Pd nanoparticles, respectively. Blue, purple, green, and red colors in (e) represent Zn, O, Pd, and Au elements, respectively.
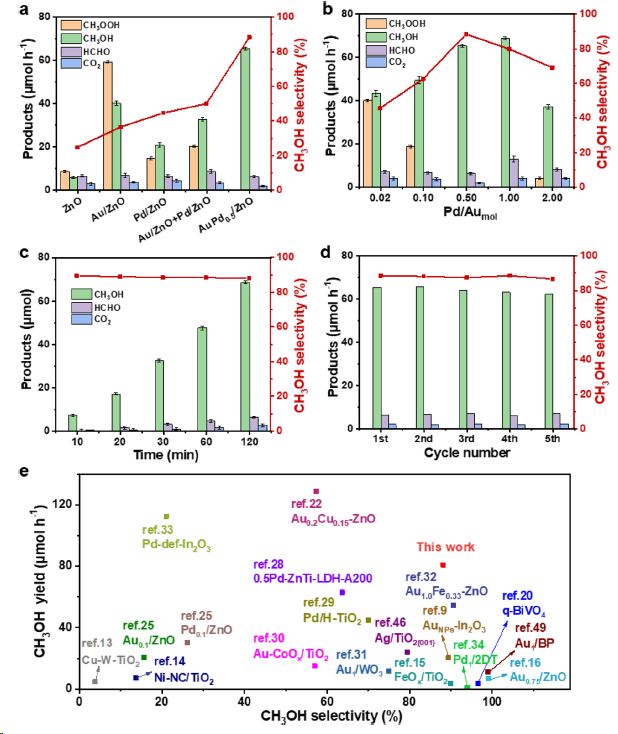
Figure 2. Photocatalytic CH4 oxidation performance over (a) ZnO, Au/ZnO, Pd/ZnO, Au/ZnO + Pd/ZnO (physical mixture) and AuPd0.5/ZnO, (b) 1.0% AuPdy/ZnO with different molar ratios of Pd to Au and investigations on (c) different reaction time and (d) cycling tests over AuPd0.5/ZnO. Standard reaction conditions: 2 mg of catalyst, 30 mL of H2O, 1 bar of O2, 29 bar of CH4, 25 °C, 0.5 h, 300 W Xe lamp. (e) Comparisons with the representative photocatalytic performances on the yield and selectivity of CH3OH. Reaction conditions: 10 mg of catalyst, 30 mL of H2O, 1 bar of O2, 29 bar of CH4, 25 °C, 2 h, 300 W Xe lamp.
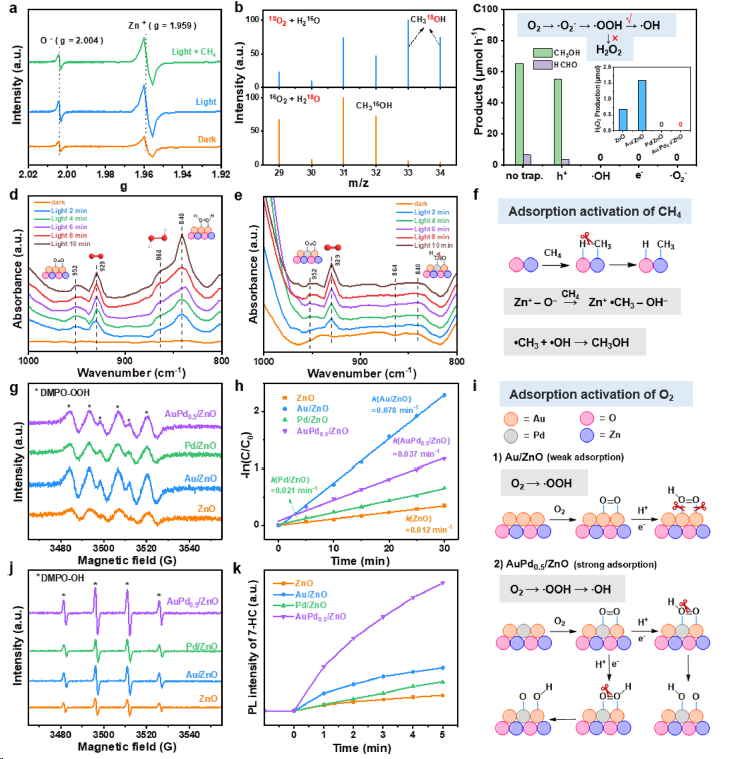
Figure 3. Mechanism of photocatalytic methane oxidation. (a) In situ EPR spectra under dark or light irradiation in an Ar atmosphere or light irradiation in a CH4 atmosphere over AuPd0.5/ZnO. (b) GC-MS results of the isotope labeling experiments in the presence of 16O2 + H2 18O or 18O2 + H2 16O. (c) Trapping experiments of active species. The inset of (c) shows H2O2 production of photocatalytic O2 reduction over different photocatalysts. In situ DRIFTS spectra of (d) Au/ZnO and (e) AuPd0.5/ZnO photocatalysts in the presence of O2 and H2O under dark or light irradiation. Schematic diagram of adsorption activation for (f) CH4 and (i) O2. In situ EPR spectra of (g) DMPO-OOH and (j) DMPO−OH for monitoring the generation of •OOH and •OH over different photocatalysts. (h) The kinetic constant of photodegradation of NBT for •OOH detection over different photocatalysts. (k) Time-dependent PL spectra of the produced 7-hydroxycoumarin for •OH detection over different photocatalysts.
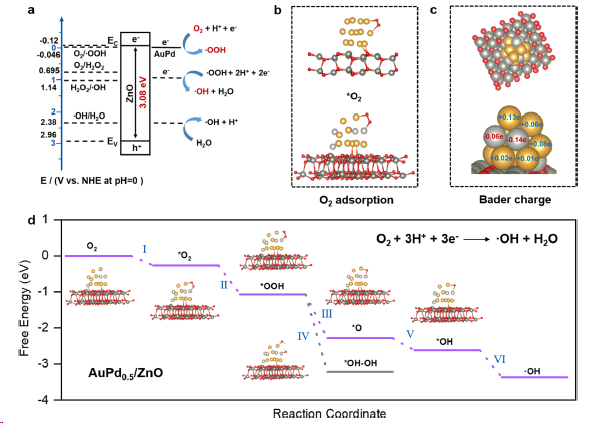
Figure 4. Mechanism of photocatalytic CH4 oxidation. (a) Band structure of AuPd0.5/ZnO with the redox potential of typical radicals. (b) Schemeof the O2 adsorption configurations on Au/ZnO (top) and AuPd0.5/ZnO (bottom). (c) Bader charge analysis of AuPd0.5/ZnO. Yellow, light gray, dark gray and red represent Au, Pd, Zn and O atoms, respectively. (d) Free-energy diagram of O2 adsorption and reduction on AuPd0.5/ZnO. Inset figure shows the adsorption models of intermediates on the surface of AuPd0.5/ZnO.
Selective Photocatalytic Oxidation of Methane to Methanol by Constructing a Rapid O2 Conversion Pathway over Au−Pd/ZnO
声明:本文仅用于学术文章转载分享,不做盈利使用,如有侵权,请及时联系小编删除。





